Tuning cation–anion interactions in ionic liquids by changing the conformational flexibility of the cation†
Abstract
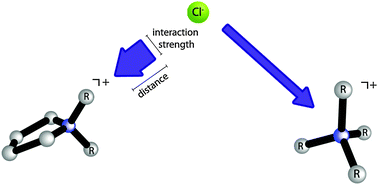
Cation–anion interactions can be tuned via the degree of cation constraint. X-ray Photoelectron Spectroscopy (XPS) experiments reveal that anion–cation based interactions may be enhanced by introducing conformational restriction into the substituent chains of quaternary ammonium cations. A larger degree of charge-transfer was observed for the constrained [C8C1Pyrr]+ cation relative to the [N6,6,6,14]+ cation.

 Please wait while we load your content...
Please wait while we load your content...