Fluorinative hydrolysis of phosphorothioic acid esters with a binaphthyl group through axis-to-center chirality transfer leading to the formation of P-chiral phosphorothioic monofluoridic acid salts†
Abstract
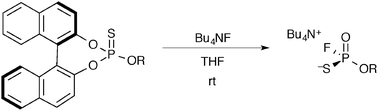
Asymmetric synthesis of P-chiral phosphorothioic monofluoridic acid ammonium salts was achieved via axis-to-center chirality transfer reactions by using phosphorothioic acid O-esters with a binaphthyl group, and the absolute stereochemistry of the salts was determined by X-ray analyses and by comparison of their CD spectra.

 Please wait while we load your content...
Please wait while we load your content...