An efficient one-pot strategy for the highly regioselective metal-free synthesis of 1,4-disubstituted-1,2,3-triazoles†
Abstract
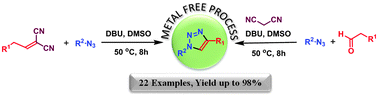
A simple and efficient metal-free methodology for the regioselective synthesis of 1,4-disubstituted-1,2,3-triazoles has been developed by applying a novel inverse electron-demand-1,3-dipolar cycloaddition approach. The practical one-pot metal-free strategy can be accomplished with various alkylidene malononitriles and aromatic azides in the presence of base.

 Please wait while we load your content...
Please wait while we load your content...