Evidence of two-state reactivity in alkane hydroxylation by Lewis-acid bound copper–nitrene complexes†
Abstract
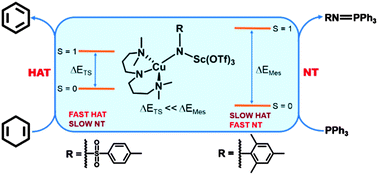
The behavior of the Lewis-acid adducts of two copper–nitrene [Cu(NR)]+ complexes in nitrene-transfer and H-atom abstraction reactions have been demonstrated to depend on the nature of the nitrene substituents. Two-state reactivity, in which a singlet ground state and a nearby triplet excited-state both contribute, provides a useful model for interpreting reactivity trends of the two compounds.
- This article is part of the themed collection: Non-Innocent Ligands

 Please wait while we load your content...
Please wait while we load your content...