Hydrogen-bonded clusters of ferrocenecarboxylic acid on Au(111)
Abstract
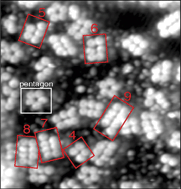
Self-assembled monolayers of ferrocenecarboxylic acid (FcCOOH) contain two fundamental units, both stabilized by intermolecular hydrogen bonding: dimers and cyclic five-membered catemers. At surface coverages below a full monolayer, however, there is a significantly more varied structure that includes double-row clusters containing two to twelve FcCOOH molecules. Statistical analysis shows a distribution of cluster sizes that is sharply peaked compared to a binomial distribution. This rules out simple nucleation-and-growth mechanisms of cluster formation, and strongly suggests that clusters are formed in solution and collapse into rows when deposited on the Au(111) surface.
- This article is part of the themed collection: Scanning Probe Studies of Molecular Systems

 Please wait while we load your content...
Please wait while we load your content...