Pd(ii)-Catalyzed arylation of unactivated methylene C(sp3)–H bonds with aryl halides using a removable auxiliary†
Abstract
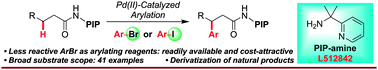
A Pd(II)-catalyzed arylation of methylene C(sp3)–H bonds in aliphatic amides directed by our newly developed PIP directing group with aryl iodides/bromides has been achieved. Arylation occurs efficiently with a broad range of aryl halides and amides.

 Please wait while we load your content...
Please wait while we load your content...