Tuning the electronic effects of aromatic phosphorus heterocycles: an unprecedented phosphinine with significant P(π)-donor properties†
Abstract
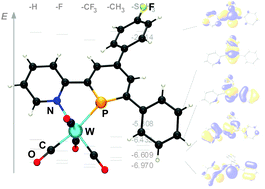
A hitherto unprecedented electronic situation has been observed for a substituted, pyridyl-functionalized phosphinine. In contrast to previous studies, this compound shows considerable π-donor properties as the result of the rather strong +M effect of the CH3S-substituent, changing the electronic properties of this low-coordinate and aromatic phosphorus heterocycle substantially.

 Please wait while we load your content...
Please wait while we load your content...