Palladium-catalyzed formal arylacylation of allenes employing acid chlorides and arylboronic acids†
Abstract
Palladium-catalyzed formal arylacylation of allenes using acid chlorides and arylboronic acids has been achieved. The reaction afforded the corresponding α,β-unsaturated ketones regio- and stereoselectively.
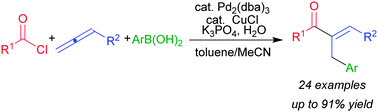

 Please wait while we load your content...
Please wait while we load your content...