Surprising acidity of hydrated lithium cations in organic solvents†
Abstract
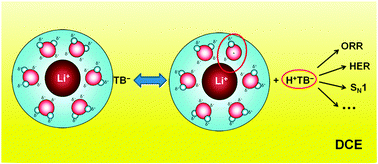
Lithium cations are shown to have a significant role in catalyzing oxygen and proton reduction along with SN1 reactions in biphasic systems. We propose that this catalytic effect is due to the surprising acidity of the hydrated cations; interactions between the cation and its surrounding solvation shell will make the constituent water molecules more acidic.

 Please wait while we load your content...
Please wait while we load your content...