Organocatalytic cascade reactions: diversity-oriented synthesis for the construction of hydroisoquinoline scaffolds†
Abstract
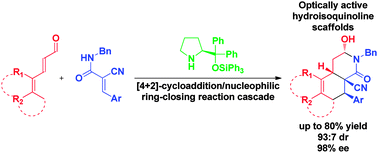
The organocatalytic enantioselective synthesis of highly functionalized hydroisoquinolines by trienamine-mediated [4+2]-cycloaddition/nucleophilic ring-closing reaction cascade sequence of cyanoacrylamides with 2,4-dienals is presented. The corresponding cycloadducts are formed in high yields and excellent stereoselectivities. Moreover, a series of transformations demonstrate the synthetic application of the obtained hydroisoquinolines.

 Please wait while we load your content...
Please wait while we load your content...