Sequential one-pot enzymatic synthesis of oligo-N-acetyllactosamine and its multi-sialylated extensions†
Abstract
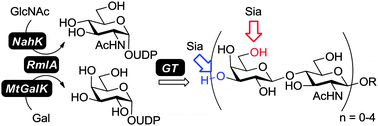
A simple and efficient protocol for the preparative-scale synthesis of various lengths of oligo-N-acetyllactosamine (oligo-LacNAc) and its multi-sialylated extensions is described. The strategy utilizes one thermophilic bacterial thymidylyltransferase (RmlA) coupled with corresponding sugar-1-phosphate kinases to generate two uridine diphosphate sugars, UDP-galactose and UDP-N-acetylglucosamine. By incorporating glycosyltransferases, oligo-LacNAcs and their sialylated analogs were synthesized.

 Please wait while we load your content...
Please wait while we load your content...