Propargylamine–isothiocyanate reaction: efficient conjugation chemistry in aqueous media†
Abstract
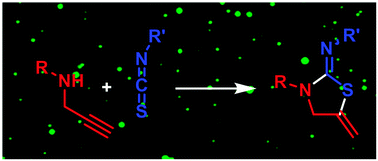
A coupling reaction between secondary propargyl amines and isothiocyanates in aqueous media is described. The reaction is high-yielding and affords cyclized products within 2–24 h. A functionalized ether lipid was synthesized in 8 steps, formulated as liposomes with POPC and conjugated to FITC under mild conditions using this method.

 Please wait while we load your content...
Please wait while we load your content...