Controlled thioamide vs. amide formation in the thioacid–azide reaction under acidic aqueous conditions†
Abstract
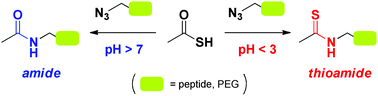
The thioacid–azide reaction and its chemoselectivity were probed with alkyl azides for a potential application to form amide bonds in aqueous solvents. Our results reveal that under acidic conditions thioamides were formed as major reaction products suggesting a competing mechanism, whereas reactions forming amides predominated at slightly higher pH values.

 Please wait while we load your content...
Please wait while we load your content...