Asymmetric semipinacol rearrangement of 2,3-allenols with N-bromo-1,8-naphthalimide†
Abstract
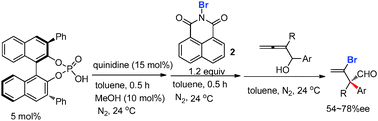
A method using quinidine and optically active binol-derived phosphoric acid as a cocatalyst to catalyze the asymmetric semipinacol rearrangement of 2,3-allenols forming optically active 3-bromo-3-enals that contain an all-carbon quaternary stereocenter has been developed. After some further treatments, the products with practical enantiomeric purity could be prepared.

 Please wait while we load your content...
Please wait while we load your content...