A novel P450-based biocatalyst for the selective production of chiral 2-alkanols†
Abstract
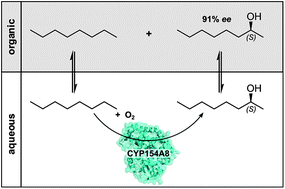
A P450 monooxygenase from Nocardia farcinica (CYP154A8) catalyses the stereo- and regioselective hydroxylation of n-alkanes, still a challenging task in chemical catalysis. In a biphasic reaction system, the regioselectivity for the C2-position of C7–C9 alkanes was over 90%. The enzyme showed strict S-selectivity for all tested substrates, with enantiomeric excess (ee) of up to 91%.

 Please wait while we load your content...
Please wait while we load your content...