Rh(iii)-Catalyzed intramolecular redox-neutral cyclization of alkenes via C–H activation†
Abstract
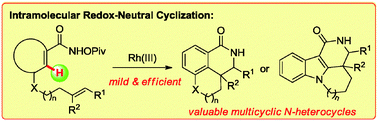
Biologically interesting fused oligocyclic lactams have been prepared via an intramolecular redox-neutral cyclization process. By the proper choice of the substrates with a wide variety of tethered olefins, the less favored C–H bond can be activated and functionalized. This C–H activation proceeds under mild conditions, obviates the need for external oxidants, and displays a broad scope with respect to the substituents.

 Please wait while we load your content...
Please wait while we load your content...