Cyclo-oligo-(1 → 6)-β-d-glucosamine based artificial channels for tunable transmembrane ion transport†
Abstract
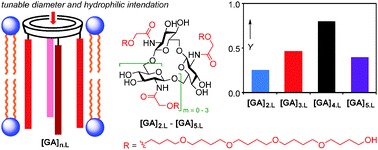
Unimolecular ion channels were designed by functionalization of a new type of cyclic oligosaccharides, cyclo-oligo-(1 → 6)-β-D-glucosamines, with pentabutylene glycol chains. Their ion transporting activity was tuned by varying oligomericity. A halide selectivity sequence, Cl− > Br− > I− was observed.

 Please wait while we load your content...
Please wait while we load your content...