Disproportionation of a 2,2-diphenyl-1-picrylhydrazyl radical as a model of reactive oxygen species catalysed by Lewis and/or Brønsted acids†
Abstract
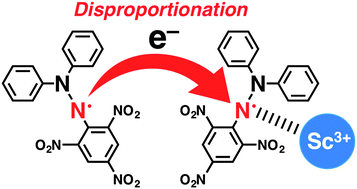
Electron-transfer disproportionation of a 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙) occurred in the presence of Sc3+ acting as a strong Lewis acid in deaerated acetonitrile. In contrast, in the case of weaker Lewis acids than Sc3+, such as Mg2+ and Li+, external protons from acetic acid were required for the disproportionation of DPPH˙ to occur.

 Please wait while we load your content...
Please wait while we load your content...