Convenient one-step construction of yne-functionalized aryl halides through domino cyclization from tetraynes†
Abstract
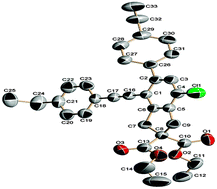
An efficient method for the construction of fused yne-substituted aryl halides by reaction of unactivated linear tetraynes with allyl halides via domino C–C coupling and formation of C–X bonds in the presence of Pd(OAc)2/PPh3 was developed.

 Please wait while we load your content...
Please wait while we load your content...