Development and validation of a microbiological assay by turbidimetry to determine the potency of cefazolin sodium in the lyophilized powder form
Abstract
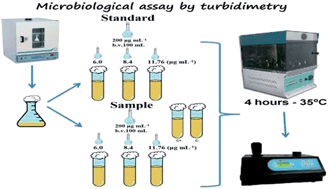
In many countries, high rates of mortality and morbidity from infectious diseases represent high social and economic costs. Cefazolin sodium is a semi-synthetic β-lactam antimicrobial for parenteral use, belonging to the first-generation cephalosporin's group. Its use in clinical practice stands out for its effectiveness as a therapeutic agent and in surgical prophylaxis, having great importance in the fight against many diseases. This paper reports the development and validation of an efficient, accurate, reproducible, and low cost microbiological assay by a turbidimetric method to quantify cefazolin in the lyophilized powder form. These requirements are essential for the analysis of this cephalosporin in the pharmaceutical industry. The assay is based on the inhibitory effect of cefazolin sodium upon the strain of Staphylococcus aureus ATCC 26923 used as the test microorganism. The method was validated according to the ICH guidelines and the results were treated by analysis of variance (ANOVA), proving to be linear (r2 = 0.9999 for the reference substance and r2 = 0.9995 for the sample), in the selected range from 6 to 11.76 μg mL−1, precise (RSD values < 2.0%), robust and accurate (99.92%). The developed method showed excellent validation results, and the statistical analysis corroborated with its assessment. Furthermore, Student's t-test showed no statistically significant difference between the proposed turbidimetric method and an UV spectrophotometry method previously validated. Thus, the validated method is able to quantify cefazolin sodium in the powder form for injectable solution, while being an economical and rapid alternative for its routine analysis in quality control.

 Please wait while we load your content...
Please wait while we load your content...