Highly sensitive colorimetric detection of HgII and CuII in aqueous solutions: from amino acids toward solid platforms†
Abstract
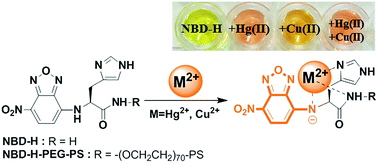
A chemosensor (NBD-H) based on an amino acid with 7-nitro-2,1,3-benzoxadiazole was used for selective detection of HgII and CuII among 15 metal ions in aqueous solutions by a colorimetric change. NBD-H sensitively differentiated HgII and CuII in aqueous solutions by a color change; a pink color for HgII and an orange color for CuII. NBD-H showed nanomolar detection limits for HgII (176 nM, R2 = 0.996) and CuII (163 nM, R2 = 0.996). The detection limit for CuII was much lower than the maximum allowable level of CuII in drinking water recommended by the U.S. EPA. The binding mode study showed that deprotonation of the NH group of NBD-H played a critical role in the binding and sensing of metal ions. NBD-H immobilized on PEG-PS resin maintained the potent binding affinity and sensing ability for the metal ions. The resin with NBD-H was recyclable for the detection of metal ions in 100% aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...