Quantitative serine protease assays based on formation of copper(ii)–oligopeptide complexes†
Abstract
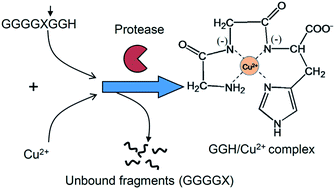
A quantitative protease assay based on the formation of a copper–oligopeptide complex is developed. In this assay, when a tripeptide GGH fragment is cleaved from an oligopeptide chain by serine proteases, the tripeptide quickly forms a pink GGH/Cu2+ complex whose concentration can be determined quantitatively by using UV-Vis spectroscopy. Therefore, activities of serine proteases can be determined from the formation rate of the GGH/Cu2+ complex. This principle can be used to detect the presence of serine protease in a real-time manner, or measure proteolytic activities of serine protease cleaving different oligopeptide substrates. For example, by using this assay, we demonstrate that trypsin, a model serine protease, is able to cleave two oligopeptides GGGGKGGH (P1) and GGGGRGGH (P2). However, the specificity constant (kcat/Km) for P2 is higher than that of P1 (6.4 × 103 mM−1 min−1vs. 1.3 × 103 mM−1 min−1). This result shows that trypsin is more specific toward arginine (R) than lysine (K) in the oligopeptide sequence.

 Please wait while we load your content...
Please wait while we load your content...