Methane–oxygen electrochemical coupling in an ionic liquid: a robust sensor for simultaneous quantification†
Abstract
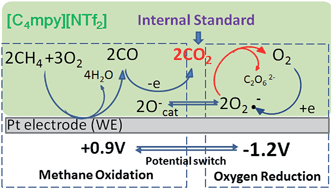
Current sensor devices for the detection of methane or natural gas emission are either expensive and have high power requirements or fail to provide a rapid response. This report describes an electrochemical methane sensor utilizing a non-volatile and conductive pyrrolidinium-based ionic liquid (IL) electrolyte and an innovative internal standard method for methane and oxygen dual-gas detection with high sensitivity, selectivity, and stability. At a platinum electrode in bis(trifluoromethylsulfonyl)imide (NTf2)-based ILs, methane is electro-oxidized to produce CO2 and water when an oxygen reduction process is included. The in situ generated CO2 arising from methane oxidation was shown to provide an excellent internal standard for quantification of the electrochemical oxygen sensor signal. The simultaneous quantification of both methane and oxygen in real time strengthens the reliability of the measurements by cross-validation of two ambient gases occurring within a single sample matrix and allows for the elimination of several types of random and systematic errors in the detection. We have also validated this IL-based methane sensor employing both conventional solid macroelectrodes and flexible microfabricated electrodes using single- and double-potential step chronoamperometry.

 Please wait while we load your content...
Please wait while we load your content...