Effective synthesis of kynurenine-containing peptides via on-resin ozonolysis of tryptophan residues: synthesis of cyclomontanin B†
Abstract
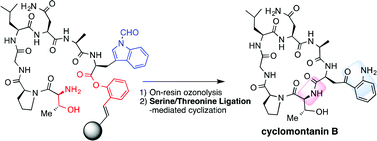
The preparation of Kyn-containing peptides is difficult, owing to the low reactivity of Kyn in the coupling reaction. In this report, Kyn-containing peptides were efficiently obtained via on-resin ozonolysis of the corresponding Trp-containing peptide. In addition, a Kyn-containing cyclic peptide, cyclomontanin B, has been synthesized by this strategy in the combination with serine/threonine ligation (STL)-mediated cyclization.

 Please wait while we load your content...
Please wait while we load your content...