Abstract
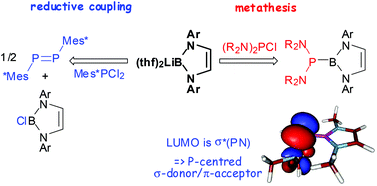
A lithio-diazaborole reacted with diamino-chlorophosphines via metathesis to yield previously unavailable phosphinoboranes bearing amino substituents at both the phosphorus and boron atoms, and with Ph2PCl and Mes*PCl2via chloride transfer and reductive PP coupling to give a chloro-diazaborole and the corresponding diphosphine or diphosphene, respectively. Diazaboroles with phenylphosphino- and PH2-substituents were nonetheless accessible via inverse metathesis upon treatment of a bromoborane precursor with phosphides PhnPH2−nM (n = 0–2, M = Li, K). The products were characterised by spectroscopic data and in most cases by single-crystal X-ray diffraction studies which show the molecules to exhibit strongly pyramidal coordination at the phosphorus atom and long BP bonds of 1.93–1.95 Å. The insensitivity of the BP distance towards substituent effects and the tolerance of large sterically induced torsional twists along the BP bond axis suggest the presence of pure single bonds without any contribution from P→B dative π-interactions. This view was confirmed by DFT studies which indicate further that the molecules lack a significant electrophilic character at boron but may act as potential σ-donor/π-acceptor ligands through the phosphorus atom.

 Please wait while we load your content...
Please wait while we load your content...