Understanding the defect chemistry of tin monoxide
Abstract
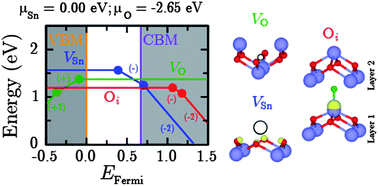
Tin monoxide has garnered a great deal attention in the recent literature, primarily as a transparent p-type conductor. However, due to its layered structure (dictated by non-bonding dispersion forces) simulation via density functional theory often fails to accurately model the unit cell. This study applies a PBE0-vdW methodology to accurately predict both the atomic and electronic structure of SnO. Empirical van der Waals corrections improve the structure, with the calculated c/a ratio matching experiment, while the PBE0 hybrid-DFT method gives accurate band gaps (0.67 and 2.76 eV for the indirect and direct band gaps) and density of states which are in agreement with experimental spectra. This methodology has been applied to the simulation of the native intrinsic defects of SnO, to further understand the conductivity. The results indicate that n-type conductivity will not arise from intrinsic defects and that donor doping would be necessary. For p-type conduction, the Sn vacancy is seen to be the source, with the 0/−1 transition level found 0.39 eV above the valence band maximum. By considering the formation energies and transition levels of the defects at different chemical potentials, it is found that the p-type conductivity is sensitive to the O chemical potential. When the chemical potential is close to its lowest value (−2.65 eV here), the oxygen vacancy is stabilized which, whilst not leading to n-type conduction, could reduce p-type conduction by limiting the formation of hole states.

 Please wait while we load your content...
Please wait while we load your content...