A blue-white-yellow color-tunable excited state intramolecular proton transfer (ESIPT) fluorophore: sensitivity to polar–nonpolar solvent ratios
Abstract
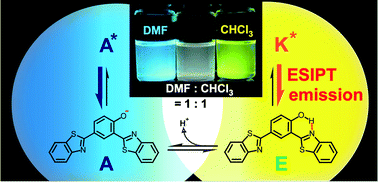
We report the emission wavelength tunability of an excited state intramolecular proton transfer (ESIPT) fluorophore, 2,4-dibenzothiazolylphenol (2,4-DBTP), and its application to white light generation. In a nonpolar solvent, yellow fluorescent 2,4-DBTP had a maximum emission around 560 nm, but did not absorb light in the blue region (400–500 nm) because of its large Stokes shift. In a polar solvent, however, a phenol proton of 2,4-DBTP involving in ESIPT is dissociated by solvent molecules, giving rise to blue emission. By dissolving the fluorophore in a polar–nonpolar solvent mixture, we observed white emission with a broad band ranging from 400 to 650 nm, a consequence of the small overlap between the absorption spectrum of the yellow-emitting form and the fluorescence spectrum of the blue-emitting form. We also successfully fabricated a white-emitting polymer film using yellow-emitting 2,4-DBTP and a representative blue fluorescent dye (perylene) as dopants. Our fluorophore will be useful for environmentally sensitive fluorescent probes and white organic light-emitting diodes.

 Please wait while we load your content...
Please wait while we load your content...