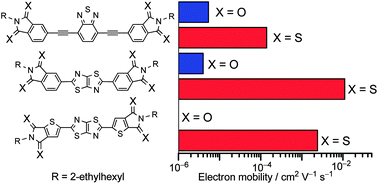
A series of new π-conjugated systems bearing arenedithiocarboxyimides (dithioimides) as electron-accepting terminal units were prepared utilizing thionation of the imide compounds in the final step of the synthesis. The thermal properties of the dithioimide compounds demonstrated that they had a weak crystallization nature, and their photophysical and electrochemical properties were significantly different from those of their imide analogs. As a result, the dithioimide compounds had narrower highest occupied molecular orbital (HOMO) – lowest unoccupied molecular orbital (LUMO) energy gaps, and lower LUMO energy levels than those of the corresponding imide compounds. Organic field-effect transistors (OFETs) based on the dithioimide compounds showed good electron-transporting characteristics. Furthermore, the observed OFET performances were dramatically improved compared to those for the crystalline films of the corresponding imide derivatives, despite their tendency to form amorphous films. This unexpected phenomenon could be attributed to the presence of strong intermolecular electronic interactions for the dithioimide compounds, which induced the construction of a non-directional charge-transport pathway. Thus, the increase in electron mobilities for the dithioimide compounds was attributed to the combined effect of the low-lying LUMO energy level and the strong intermolecular electronic interactions in the solid state. Organic photovoltaics based on poly(3-hexylthiophene) as the hole-transporting material and the dithioimide compounds as the electron-transporting material exhibited poorer performances due to the high miscibility between the two compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...