Synthesis and characterization of some novel tetrazole liquid crystals†
Abstract
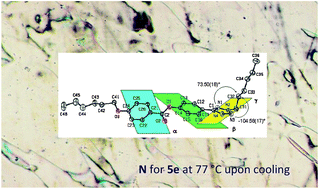
The synthesis and characterization of new 4-[(2-alkyl)-2H-tetrazol-5-yl]phenyl 4-alkyloxybenzoates (5a–i) and 5-{4-[(4-substituted)benzyloxy]phenyl}-2-alkyl-2H-tetrazoles (7a–e) are reported. The reported

 Please wait while we load your content...
Please wait while we load your content...