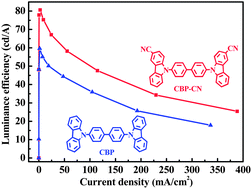
N,N′-Dicarbazolyl-4,4′-biphenyl (CBP) is one of the most successful uni-polar host materials for phosphorescent organic light-emitting diodes (PhOLEDs). We report the synthesis and properties of one novel CBP derivative, CBP-CN, with two cyano groups (CN) at the 3-site of carbazole rings. The strong electron-withdrawing CN group was introduced with the expectation to promote electron-injecting/-transporting abilities and to achieve bipolar features for CBP-CN. In comparison with the parent CBP, CBP-CN possesses lowered HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) levels and dramatically increased Tg (glass transition temperature, 162 °C), but unaltered HOMO–LUMO band gap and triplet energy (2.69 eV). Green and red PhOLEDs were fabricated with CBP-CN as hosts for traditional iridium phosphors. The maximum luminance efficiency (ηL) of 80.61 cd A−1 (23.13%) was achieved for the green PhOLED, and 10.67 cd A−1 (15.54%) for the red one, which represent efficiency increases of 25–33% compared with those of the best devices with CBP host and are even among the best data for phosphorescent OLEDs reported so far. The theoretical calculation and the carrier-only devices investigation confirmed that the electron-injecting/-transporting character and the bipolar nature of CBP-CN should be responsible for the performance enhancements.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...