Electrocatalytic activity of well-defined and homogeneous cubic-shaped Pd nanoparticles†
Abstract
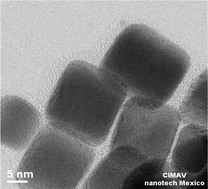
Well-ordered, homogenous cube-shaped highly ordered Pd, without other geometries, was obtained by chemical reduction in aqueous media by employing ascorbic acid, polyvinylpyrrolidone and sodium bromide as the reducing agent, surfactant and additive, respectively. The X-ray diffraction (XRD) patterns exhibited a face-centred cubic structure with an average crystallite size of 11.5 nm. Transmission electron microscopy (TEM) images showed a homogeneous distribution of cubic Pd nanoparticles (namely Pd nanocubes) with a preferential (200) crystallographic plane and a particle size of approximately 10.6 ± 1.2 nm. The electrocatalytic activity of the Pd nanocubes was evaluated in terms of the current density attributed to the oxidation reaction of the following three common fuels: methanol, ethanol and formic acid. The results obtained showed that the current density achieved with the Pd nanocube electrocatalyst is 4, 3 and 3.5 (12, 10 and 8.7 mA mg−1 cm−2, respectively) times higher than that reached with commercial Pd at similar oxidation potential values. Furthermore, the Pd nanocube catalyst exhibited higher catalytic activity for formic acid oxidation than that reported for Pd-based materials. The oxygen reduction reaction using the Pd nanocubes in basic media was also tested.

 Please wait while we load your content...
Please wait while we load your content...