Cell degradation of a Na–NiCl2 (ZEBRA) battery
Abstract
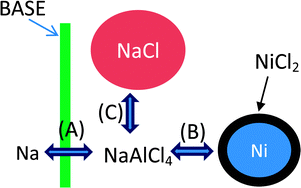
In this work, the parameters influencing the degradation of a Na–NiCl2 (ZEBRA) battery were investigated. Planar Na–NiCl2 cells using the β′′-alumina solid electrolyte (BASE) were tested with different C-rates, Ni/NaCl ratios, and capacity windows, in order to identify the key parameters for the degradation of the Na–NiCl2 battery. The morphology of NaCl and Ni particles was extensively investigated after 60 cycles under various test conditions using a scanning electron microscope. A strong correlation between the particle size (NaCl and Ni) and battery degradation was observed in this work. Even though the growth of both Ni and NaCl can influence the cell degradation, our results indicate that the growth of NaCl is a dominant factor in cell degradation. The use of excess Ni seems to play a role in tolerating the negative effects of particle growth on degradation since the available active surface area of Ni particles can still be sufficient even after particle growth. For NaCl, a large cycling window was the most significant factor, of which effects were amplified with decrease in the Ni/NaCl ratio.

 Please wait while we load your content...
Please wait while we load your content...