Polysulfone-based anion exchange membranes demonstrate excellent chemical stability and performance for the all-vanadium redox flow battery†
Abstract
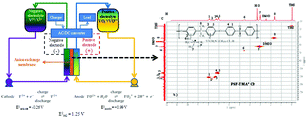
A polysulfone-based anion exchange membrane functionalized with quaternary benzyl trimethylammonium groups (PSF-TMA+) demonstrated a 40-fold reduction in vanadium(IV) permeability when compared to a Nafion® membrane. Comprehensive 2D NMR (COSY and heteronuclear quantum correlation spectroscopy) studies verified that PSF-TMA+ remained chemically stable even after exposure to a 1.5 M vanadium(V) solution for 90 days. Excellent energy efficiencies (85%) were attained and sustained over several charge–discharge cycles for a vanadium redox flow battery prepared using the PSF-TMA+ separator.

 Please wait while we load your content...
Please wait while we load your content...