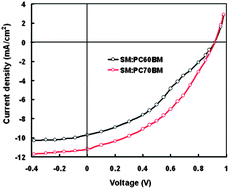
A new A–D–A small molecule (SM) based on a 3,6-dithienylcarbazole donor unit at the center and nitrophenyl acrylonitrile acceptor units as the terminal ends was synthesized by the Pd catalyzed Stille coupling reaction for application as a donor material in the organic bulk heterojunction solar cells. The SM possesses a low lying HOMO energy level at −5.34 eV and optical band gap of 1.74 eV. The photovoltaic performance of the SM was investigated with PC60BM and PC70BM as acceptors cast from THF with 3% CN additive and without additive. The power conversion efficiency (PCE) of the organic solar cells based on SM:PC60BM cast from THF solvent reached 1.94% with a high Voc of 0.94 V, a Jsc of 6.08 mA cm−2 and FF of 0.34, where as the PCE of SM:PC70BM cast from THF solvent was 2.65% with a Voc of 0.96 V, a Jsc of 7.26 mA cm−2 and FF of 0.38%, under the illumination intensity of 100 mW cm−2. The higher PCE of the organic solar cells based on PC70BM as an electron acceptor has been attributed to its strong absorption in the visible region than PC60BM. The PCE of the organic solar cells has been further improved to 3.76% and 4.96% for SM:PC60BM and SM:PC70BM, respectively, cast with CN–THF solvent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...