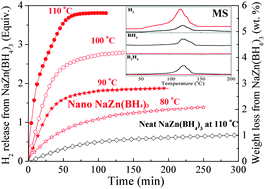
In the present work, the decomposition behaviour of NaZn(BH4)3 nanoconfined in mesoporous SBA-15 has been investigated in detail and compared to bulk NaZn(BH4)3 that was ball milled with SBA-15, but not nanoconfined. The successful incorporation of nanoconfined NaZn(BH4)3 into mesopores of SBA-15 was confirmed by scanning electron microscopy, transmission electron microscopy, energy dispersive X-ray spectroscopy, 11B nuclear magnetic resonance, nitrogen absorption/desorption isotherms, and Fourier transform infrared spectroscopy measurements. It is demonstrated that the dehydrogenation of the space-confined NaZn(BH4)3 is free of emission of boric by-products, and significantly improved hydrogen release kinetics is also achieved, with pure hydrogen release at temperatures ranging from 50 to 150 °C. By the Arrhenius method, the activation energy for the modified NaZn(BH4)3 was calculated to be only 38.9 kJ mol−1, a reduction of 5.3 kJ mol−1 compared to that of bulk NaZn(BH4)3. This work indicates that nanoconfinement within a mesoporous scaffold is a promising approach towards stabilizing unstable metal borohydrides to achieve hydrogen release with high purity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...