Hierarchically mesoporous silica single-crystalline nanorods with three dimensional cubic Fm-3m mesostructure†
Abstract
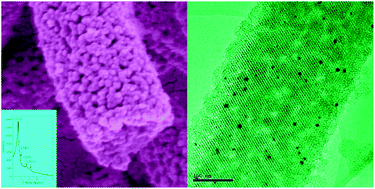
Hierarchically mesoporous silica nanorods with well-ordered cubic Fm-3m mesostructure were fabricated for the first time under basic conditions by using cationic surfactant cetyltrimethylammonium bromide (CTAB) as a template with poly(acrylic acid) (PAA) and triblock copolymer Pluronic P123 (PEO20PPO70PEO20) as co-templates. Due to the electrostatic interaction between CTAB and PAA, they would co-assemble to form complex colloids. The nonionic surfactant P123 would also be incorporated into the complex. The CTA/PAA/P123 complex colloids exhibited the morphology of nanorods, and the morphology of the final product inherited from the complex. The ordered mesopores (∼3 nm) of hierarchically mesoporous silica were connected with the secondary mesopores, whose average pore size was about 20 nm. Interestingly, the existence of the secondary mesopores did not disturb the ordered mesostructure of the nanorods and thus all the nanorods remained as single-crystalline mesoporous silica crystals. By varying the average molecular weight of the triblock copolymers, we could obtain hierarchically mesoporous silica with 2-D hexagonal mesostructure, which had a high surface area (∼980 m2 g−1) and large pore volume (∼1.3 cm3 g−1). Au-supported hierarchically mesoporous silica nanorods exhibited much higher reaction rate than that of Au-supported MCM-41 in catalytic reduction of 4-nitrophenol, as a result of its unique hierarchically mesoporous structure.

 Please wait while we load your content...
Please wait while we load your content...