Multifunctional system based on hybrid nanostructured rod formation, for sensoremoval applications of Pb2+ as a model toxic metal†
Abstract
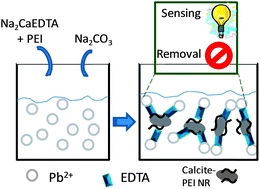
A new and simple multifunctional system based on hybrid calcite–poly(ethyleneimine) (PEI) nanostructured rod (NR) formation, which shows as a proof-of-concept the sensing and removal (sensoremoval concept) of Pb2+ as a model toxic metal for environmental applications, has been developed. The addition of Na2CaEDTA and Na2CO3 solutions to Pb2+ contaminated water leads to the crystallization of calcite–PEI NRs. These NRs are composed of self-assembled hexagonal plate-like shapes of around 450 nm. The formed calcite–PEI NRs in suspension permit Pb2+ detection, through a simple turbidimetric measurement, and at the same time act as a Pb2+ remover. This sensing and removal system is able to detect up to 1 ppm Pb2+ (1–1000 ppm linear range) and reaches an adsorption capacity of 240 mg Pb2+ per g of NRs, as evaluated using a 342 ppm initial Pb2+ concentration at pH 4 after 30 min incubation time. The maximum Pb2+ removal capacity reported here is higher than other capacities reported previously, using materials that are not as cheap and biodegradable as calcite. The Pb2+ detection range found for this system is suitable to evaluate levels of metal contamination from industrial wastewater of around 1–100 ppm. This system based on an advanced biodegradable material constitutes a useful tool as a proof-of-concept in the design of future multifunctional platforms for the development of integrated environmental technologies with sensing and remediation functions.

 Please wait while we load your content...
Please wait while we load your content...