Synthesis and photocatalytic hydrogen production of a novel photocatalyst LaCO3OH†
Abstract
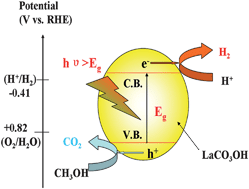
A novel orthorhombic LaCO3OH photocatalyst is synthesized by a facile hydrothermal method. The prepared LaCO3OH sample exhibits high photocatalytic activity for hydrogen evolution from aqueous methanol solutions under UV light irradiation, which is about 3 times higher than that of anatase TiO2. The Mott–Schottky plot shows the VCB of the prepared LaCO3OH of −0.87 V vs. RHE at pH 7. Theoretical calculations of the electronic structures for LaCO3OH reveal that the top of the valence band (VB) and the bottom of the conduction band (CB) are mainly composed of O 2p and La 4f states, respectively. The superior photocatalytic performance of LaCO3OH could be ascribed predominantly to the high reduction potential of photoinduced electrons. A possible mechanism for the hydrogen evolution over LaCO3OH is proposed. This work highlights the potential application of lanthanum-based materials in the field of energy conversion.

 Please wait while we load your content...
Please wait while we load your content...