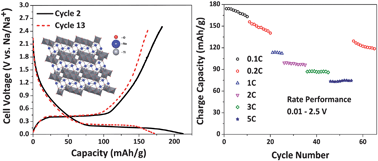
We report here the electrochemical properties of Na2Ti3O7, a potential non-carbon based, low-voltage anode material for room temperature sodium ion battery applications. A solid-state route was used to prepare Na2Ti3O7. Further, XRD, SEM, TEM, HRTEM, SAED, XPS and EDX techniques were used to characterize the material. The Na/Na2Ti3O7 cell displayed a charge capacity of 177 mA h g−1 at 0.1 C rate. High rate and long term cyclic performance at different rates showed relatively stable storage capacities. Surprisingly, if the lower cut-off voltage is altered, the appearance of a new charge plateau is seen, with no apparent change in the discharge behaviour. The kinetics of sodium insertion and extraction are discussed utilizing CV and EIS techniques. We also report the sodium chemical diffusion coefficient of the Na2Ti3O7/CB electrode estimated using GITT.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...