Interpolyelectrolyte complexes based on hyaluronic acid-block-poly(ethylene glycol) and poly-l-lysine†
Abstract
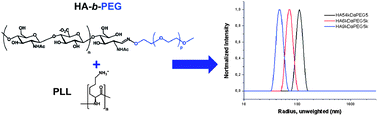
The preparation of spherical, nanosized interpolyelectrolyte complexes by the interaction of hyaluronic acid-block-poly(ethylene glycol) (HA-b-PEG) with poly-L-lysine (PLL) at a stoichiometric charge-to-charge ratio is described. The complexation was studied by dynamic light scattering and

 Please wait while we load your content...
Please wait while we load your content...