Pd(ii)-catalyzed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds using a removable directing group: efficient synthesis of alkyl ethers†
Abstract
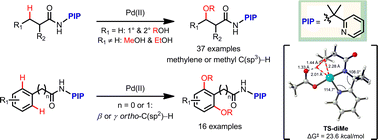
The Pd(II)-catalyzed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds using a new bidentate directing group has been developed. Alkoxylation occurs selectively at the β position with broad substrate scope and high tolerance of functional groups (

 Please wait while we load your content...
Please wait while we load your content...