Mechanochemically driven solid-state Diels–Alder reaction of graphite into graphene nanoplatelets†
Abstract
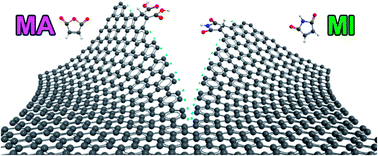
A mechanochemically driven solid-state Diels–Alder reaction is demonstrated via dry ball-
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)

 Please wait while we load your content...
Please wait while we load your content...