Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst†
Abstract
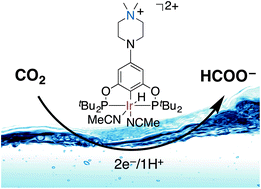
A water-soluble Ir PCP-type pincer catalyst was developed to reduce CO2 to formate electrocatalytically in water with high efficiency and selectivity. Formate is the only reduced carbon product, formed in 93% Faradaic yield with no formation of CO. A small fraction of “background” H2 (ca. 7%) is directly produced at the electrode by solvent reduction. Detailed kinetic information relevant to the catalysis was obtained. The high selectivity for formate production over H2 originates from the aqueous stability of Ir dihydride species, the active species for hydride reduction of CO2. Under neutral pH, the Ir pincer complex does not catalyze the reduction of protons to H2 making water a viable solvent for use with this catalyst system. Addition of small amounts (ca. 1%) of acetonitrile reduces the over-potential and renders the catalysis sustainable. Mechanistic studies suggest that acetonitrile is a key ancillary ligand that ionizes formate effectively preventing catalyst deactivation.

 Please wait while we load your content...
Please wait while we load your content...