Spatially controlled surface immobilization of nucleophiles via trapping of photo-generated thioaldehydes†
Abstract
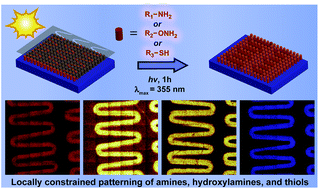
A novel and very simple photochemical strategy that utilizes light as a facile means to provide spatio-temporal control for the direct covalent immobilization of nucleophiles is presented. The concept is based upon the efficient trapping of photogenerated

 Please wait while we load your content...
Please wait while we load your content...