Gold–allenylidenes – an experimental and theoretical study†
Abstract
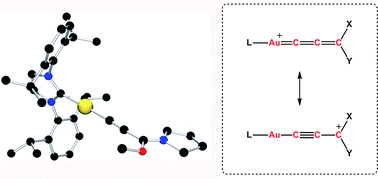
Herein we describe the isolation and characterisation of the first “gold–allenylidene” complexes [Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2]+ X− (R = N(CH2)n, OMe; n = 3, 4; X− = OTf−) and present a bonding model for these species based on an experimental and theoretical analysis. Heteroatom stabilisation (oxygen and
CR2]+ X− (R = N(CH2)n, OMe; n = 3, 4; X− = OTf−) and present a bonding model for these species based on an experimental and theoretical analysis. Heteroatom stabilisation (oxygen and

 Please wait while we load your content...
Please wait while we load your content...