Systematic modulation and enhancement of CO2 : N2 selectivity and water stability in an isoreticular series of bio-MOF-11 analogues†
Abstract
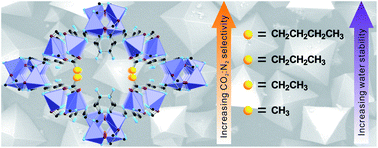
An isoreticular series of cobalt-adeninate bio-MOFs (bio-MOFs-11–14) is reported. The pores of bio-MOFs-11–14 are decorated with acetate, propionate, butyrate, and valerate, respectively. The nitrogen (N2) and carbon dioxide (CO2) adsorption properties of these materials are studied and compared. The isosteric heats of adsorption for CO2 are calculated, and the CO2 : N2 selectivities for each material are determined. As the lengths of the aliphatic chains decorating the pores in bio-MOFs-11–14 increase, the BET surface areas decrease from 1148 m2 g−1 to 17 m2 g−1 while the CO2 : N2 selectivities predicted from ideal adsorbed solution theory at 1 bar and 273 K for a 10 : 90 CO2 : N2 mixture range from 73 : 1 for bio-MOF-11 to 123 : 1 for bio-MOF-12 and finally to 107 : 1 for bio-MOF-13. At 298 K, the selectivities are 43 : 1 for bio-MOF-11, 52 : 1 for bio-MOF-12, and 40 : 1 for bio-MOF-13. Additionally, it is shown that bio-MOF-14 exhibits a unique molecular sieving property that allows it to adsorb CO2 but not N2 at 273 and 298 K. Finally, the water stability of bio-MOFs-11–14 increases with increasing aliphatic chain length. Bio-MOF-14 exhibits no loss of crystallinity or porosity after soaking in water for one month.

 Please wait while we load your content...
Please wait while we load your content...