A surface site interaction model for the properties of liquids at equilibrium†
Abstract
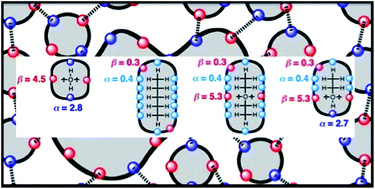
The electrostatic solvent competition model developed to estimate solvent effects on solution phase association constants for formation of 1 : 1 complexes between two solutes has been extended to provide a general treatment of intermolecular interactions in the liquid state. The interactions of a molecule with its solvation shell are described by a set of surface site interaction points (SSIPs). An SSIP represents a molecular surface area of 9.5 Å2, a volume of 5 Å3, and is characterised by an electrostatic interaction parameter, εi, obtained from the molecular electrostatic potential surface calculated in the gas phase or from functional group H-bond parameters experimentally determined in solution. A liquid is treated as an ensemble of SSIPs that interact with a probability governed by the sum of the electrostatic interaction energy, given by εiεj, and a constant van der Waals term of −5.6 kJ mol−1. The speciation of SSIP contacts is determined as a Boltzmann-weighted population of states, and this allows calculation of a variety of thermodynamic properties of solutions. The model assumes that unbound states are possible for SSIPs due to the large amount of void space present in a liquid. This provides a straightforward thermodynamic connection between different phases, because unbound states have the same chemical potential in different environments. The free energy of transfer of a molecule between two different liquid phases is calculated as the sum of a binding energy, which is a measure of the total interaction of all SSIPs in the molecule with the liquid, and a confinement energy, which is a measure of the entropic cost of confining the molecular SSIPs to that phase. Calculated liquid–liquid transfer free energies agree with experiment (±1 to 3 kJ mol−1) for a collection of alkanes, ethers, alcohols and water. The calculations provide insight into the molecular basis of the hydrophobic effect, the origin of the difference in H-bond populations in water and alcohols, and the differences between 1-octanol and n-hexadecane partition coefficients as measures of hydrophobicity. Solvent effects on association constants for formation of 1 : 1 H-bonded complexes are also reproduced by the calculations (±0.5 log units). In addition to comparison with experimental thermodynamic data, this model can also be validated using spectroscopic data on the speciation of different H-bonded states in solution.
- This article is part of the themed collection: Physical Chemistry

 Please wait while we load your content...
Please wait while we load your content...