Small molecule competition experiments were performed to determine whether Ni-catalyzed Kumada cross-coupling reactions proceed through an intramolecular oxidative addition. Indeed, preferential intramolecular oxidative addition was observed for all four complexes when stoichiometric quantities of competitive agent were present. At higher concentrations of competitive agent, the intramolecular pathway was still preferred when bidentate, electron-rich ligands were utilized, suggesting that these ligands promote the formation and reactivity of the key intermediate. To determine whether a similar pathway is involved in the polymerizations, (4-bromo-2,5-bis(hexyloxy)phenyl)magnesium bromide was polymerized in the presence and absence of competitive agent. The number-average molecular weights were lower and the molecular weight distributions were broadened substantially when competitive agent was present, consistent with the presence of competing intermolecular pathways. Because bidentate, electron-rich ligands suppressed these undesired intermolecular reactions, these ligands should lead to improved polymerization catalysts.
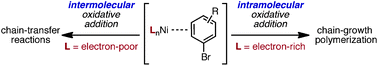
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
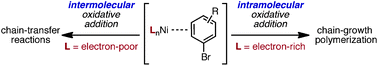

 Please wait while we load your content...
Please wait while we load your content...