Long-range metal–ligand bifunctional catalysis: cyclometallated iridium catalysts for the mild and rapid dehydrogenation of formic acid†
Abstract
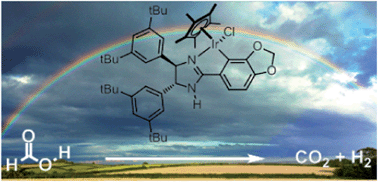
Formic acid (HCO2H) is an important potential hydrogen storage material, which, in the presence of appropriate catalysts can be selectively dehydrogenated to give H2 and CO2. In this work, well defined N^C cyclometallated iridium(III) complexes based on 2-aryl imidazoline ligands are found to be excellent catalysts for the decomposition of HCO2H–NEt3 mixtures to give H2 and CO2 under mild conditions with high turnover frequencies (up to 147 000 h−1 at 40 °C) and essentially no CO formation. The modular structures of these catalysts have allowed for the construction of structure–activity relationships for the complexes, leading to the rational optimisation of the catalyst structure with respect to both the rate of H2 production and catalyst lifetime. In particular, the presence of the remote γ-NH unit in the ligand is shown to be essential for catalytic activity, without which no reaction occurs. Mechanistic studies suggest that the dehydrogenation is rate-limited by the step of hydride protonation, which is made feasible by the γ-NH unit via an unusual form of long-range metal–ligand bifunctional catalysis involving formic acid-assisted proton hopping.

 Please wait while we load your content...
Please wait while we load your content...