One-pot three-component sulfone synthesis exploiting palladium-catalysed aryl halide aminosulfonylation†
Abstract
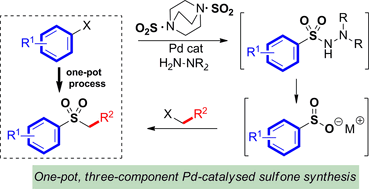
A palladium-catalysed aminosulfonylation process is used as the key-step in a one-pot, three-component sulfone synthesis. The process combines aryl-, heteroaryl- and alkenyl iodides with a sulfonyl unit and an electrophilic coupling fragment. The sulfonyl unit is delivered in the form of an aminosulfonamide, which then serves as a masked sulfinate. The sulfinate is combined, in situ, with an electrophilic coupling partner, such as a benzylic, allylic or alkyl halide, an electron-poor arene, or a cyclic epoxide, to provide the corresponding sulfone products in good to excellent yields. The mild reaction conditions and use of commercially available reaction components allows the easy preparation of a broad range of sulfones featuring a variety of functional groups. The process obviates the need to employ thiol starting materials, and oxidative operations.

 Please wait while we load your content...
Please wait while we load your content...